INTRODUCTION
Amla (Emblica officinalis Gaertn.), also known as “Indian Gooseberry” belongs to the family Euphorbiaceae.
It is one of the most important fruit of tropical and sub tropical region. Amla is thought to be a native of India, Ceylon, Malaysia and China.
India is the principal Amla growing country and Uttar Pradesh (Pratapgarh, Varanasi, Azamgarh, Sultanpur, Faizabad, Raibareli and Agra Districts) is the leading production area. It is also grown in Gujarat, Maharastra, Rajasthan and Haryana. In view of its diverse uses its cultivation is increasing very fast.
Amla fruit has played an important therapeutic role from time immemorial and is frequently recommended for its synergistic effects in both the Ayurvedic and Unani systems of medicines.
Almost all parts of the tree are used medicinally but fruit is most useful part. Fruit is used fresh or dry. Dried fruit is useful in chronic Dysentery, Haemorrhage, Diabetes, Bronchitis, Fever, Diarrhoea, Dyspepsia, Cough, Jaundice, Anemia, Piles disease associated with blood and dermatitis. Amla fruit is used in various diseases of the heart, brain and liver. Amla fruit has also antiageing factor.
Amla is probably the only fruit to fill the deficiency of astringent food recommended by both Ayurvedic and Unani system of medicines for balanced diet and sound health. Fruit is the cheapest source of Vitamin C and fair source of Vitamin B, and B2.
Fruit is sour and astringent in taste, hence it is not consumed in fresh form as table fruits. It is consumed mainly in processed form. The high nutritive and therapeutic value of this fruit offers great potentiality for processing in to a number of value added products which have better scope for both internal and external market.

VARIETIES
The ideal varieties for quality products are Banarasi, Chakaiya, Amrit (NA-6), Neelam (NA-7), Krishna (NA-5), and Kanchan (NA-4). Banarasi is reputed for its large sized fruits and has good keeping quality so it is suitable for processing.
HARVESTING
Amla fruits becomes ready for harvesting from November to February. The fruits should be picked at full maturity.
UTILIZATION
Presently Amla products fall in to three broad categories:-
(a) As Medicinal Products
Chyavanpras, Triflachurn, Herbal Syrup, Amla powder etc.
(b) As Food Products
Amla pulp, Preserve, Candy, Jam, Syrup, R.T.S., Shreds, Pickles, Squash, Sauce and wine etc.
(c) As Cosmetic Products
Shampoo, Hair oil, Hair dye etc.
FOOD VALUE
Amla has long held a place of honour in Indian medicine and products made out of it. Main constituents of fruit are carbohydrate, fat, protein, acid, minerals, vitamins, fibre and tannin.
NUTRITIVE VALUE OF FRESH AMLA PER 100 g (Reference : Fruits, NIN, ICMR)
Vitamin C : 600 mg
Vitamin A : 151 IU
Phosphorus : 20 mg
Calcium : 50 mg
Iron : 1.2 mg
HISTORY OF WINE
The history of wine is as old as the human itself. Preparation of wine was a traditional practice, Perhaps, it was left to Louis Pasteur to discover that alcoholic fermentation is due to yeast Fermentation predates recorded history. Honey and grains were probably fermented as household beverages before grapes. The early wine industry of the Fertile Crescent, about 3500 B.C., spread to Hungary, Germany and France and in the post-Columbus period to the Americas and South Africa. The Romans advanced the art of wine making, but it was an industry of large risks due to spoilage until the mid-nineteenth century. The research of Louis Pasteur revolutionized the wine industry.
Improvement in wine technology was achieved using information on other branches of science like microbiology, bio-chemistry, Bio- engineering, etc. Even today, wine is produced in certain parts of the world in the old traditional way, Countries which are producing wine today borrowed the technology from France. But the technology was modified to suit the raw materials available in their countries.
DEFINITION OF WINE
Wine can be defined as grape juice which has undergone alcoholic fermentation. It is an accepted convention that when other fruits are fermented and wine is produced, the name of the fruit is prefixed.
Sometimes, the word “wine” unqualified signifies the fermented juice of apples generally known as “Cider” or “Hard Cider” and that of pear as “Perry”, but they may also be called “Apple wine” and “Pear Wine”.
TYPES OF WINE
Wine is of different varieties depending upon the content of alcohol and methodology of preparation but Frazier (1974) defined the following wines:
- STILL WINE
Still wine is that wine which does not retain their carbon dioxide produced during the process of fermentation.
- SPARKLING WINE
Sparkling wine is a type of wine containing considerable amount of carbon dioxide produced during fermentation or from added carbon dioxide or added sugar from malolactic fermentation
- CARBONATED WINE
Carbonated wine is a type of wine, which is carbonated by artificial means and have carbon dioxide under certain limit of pressure.
- DRY WINE
Dry wine is a type of wine, which contains no fermentable sugar
- SWEET WINE
Wine having sugar left or added and usually contains 11-16% of alcohol and the minimum percentage of alcohol can go as low as 7%.
- FORTIFIED WINE
Fortified wine is the wine, which contains distillate of wine, which is popularly known as “Wine Spirit” or “Brandy”. The fortified wine generally contains 19-21% alcohol (v/v).
- TABLE WINE
Table wine is also a kind of wine, which have comparatively low content of alcohol and little or no sugar.
- DESSERT WINE
Dessert wine is a fortified sweet wine having minimum acidity 0.25% and alcohol range 19.5-21.0%,
- FRENCH DRY SHERRY
French dry sherry is made from grapes having higher sugar content, as a result of being dried out by an inoculating grey mold Botrytis cinerea, they yield a wine with high content of alcohol.
- SPANISH SHERRY
The Spanish sherry supports the growth of yeast, presumably of one or more species of Saccharomyces, while the wine being racked in partially filled barrels. This yeast growth or “Flow” imparts a special bouquet and flavor to the wine.
IMPORTANCE OF WINE
Wine represented a safe and healthful beverage (Amerine & Cruess, 1960). It also provided calories and vitamins. At a time when food was not the best, wine was an important food adjunct. During periods when life was often strenuous it offered relaxation and a very real surcease from pain
BIOCHEMISTRY OF ALCOHOLIC FERMENTATION
Alcoholic beverage has alcohol percentage in the form of ethyl alcohol (C₂H₂OH). Generally the name of wine is on its fruit. Ethyl alcohol produced by fermentation of any carbohydrate containing fermentable sugar in the form of mono, di, poly saccharide. According to Gay-Lussac egn. (1810) “Wine fermentation is basically the transformation of the various sugars of grapes by yeast under anaerobic condition in to ethanol, carbon dioxide and small amount of by products” D-glucose and D-fructose the two principal sugars of grape juice yield essentially equi-moler proporation of ethanol and carbon dioxide
C12H22O11 + H₂O
Invertase
C6H12O6 + C6H12O6
Zymase C6H12O6 + Yeast (Saccharomyces cerevisiae) 2C₂H₂OH+2CO2
In the fermentation process the catabolism of sugar is an oxidative process which result in production of ethyl alcohol.
The alcoholic fermentation taken place in the yeast call by series of reaction usually referred to as the Embden Meyerhaf-Parnas pathway (Sols et. al. 1971). The overall reaction-
Glucose Glucose di phosphate
2 Triglyceric phosphate
2 Phospho acetic acid
2 Alcohol-2 Acetaldehyde +
2 Pyruvic acid
The percentage of alcohol will depend upon the “Brix (T.S.S.) of the crushed material or must be multiplied by a factor 0.57 (R.P. Srivastava). The formation of several by product and conversion of about 1% of the sugar in to yeast call mass account for the lower yield Compounds normally recognised as bi-product alcoholic fermentation of grape are the following (percentage are based on fermentable sugar transformed). Glycerol (2-2.5%), acetic acid (0.05-0.65%), acetaldehyde (0.01-0.04%), 2,3, butanediol (0.06-0.1%) The level of all these bi- products is strongly influenced by yeast strain and environmental condition specially temperature in flavor formation. Most enologists agree that more bouquet is formed in a wine by a long slow fermentation at a low temperature than by short rapid fermentation at a temperature nearest to the maximum temperature and produce more aromatic compounds than at higher temperature.
AIM OF PROJECT
Amla is a perishable commodity and needs its quick disposal. Heavy turnover for produce in the market creates glut and the growers are bound to sell their produce at a non remunerative price. Though Amla fruit is being tried and processed in the preparation of different products like pulp, preserve, candy, jam, syrup, shreds, pickles, squash and sauce due to its attractive flavor, acidity and astringency attributes it can also be used in the preparation of alcoholic beverage (wine) This will help the industry as well as growers for providing them a remunerative source in glut period.
Amla wine should be regarded as food stuff because in the body it acts as a concentrated source of energy. It will have all the constituents of Amla fruits (Calcium, Phosphorous, Iron, Nicotinic Acid & Vitamin-C) in addition to alcohol and Vitamin-B complex.
Amla wine will enhance wound healing due to high Vitamin-C content. It retards ageing process and reforms degenerated somatic cells. Unlike most foods alcohol of Amla wine can be absorbed by the body without prior digestion and thus it will provide a source of quickly available energy and it may be used for this purpose in emergency. Alcohol of Amla wine is almost completely absorbed during its passing through the body mainly in small intestine and also through the walls of stomach.
Besides the absorption of alcohol in the body it is also possible to take some tannin, micronutrients and other natural antioxidants during absorption. It will enhance the metabolic process and the whole phenomenon will help and work as antiageing factor.
After absorption the alcohol is distributed in the body through the body system and there after it is broken down in a series of oxidative steps with liberation of energy. Thus preparation of Amla wine will be a very fruitful process.
RESEARCH PROJECT
PREPARATION OF AMLA WINE: STANDARDISATION OF A METHODOLOGY
Amla (Emblica officinalis Gaertn.) has acquired world wide popularity for its significant nutritional and medicinal properties. It is cultivated throughout the India but grown as a commercial crop in Uttar Pradesh.
Amla is being used for a variety of ailments in the Indian system of medicine since antiquity. It has been reported to have mild antibacterial, pronounced expectorant, antiviral and cardio tonic activity
The importance of this fruit is also due to its high contents of gallotanic acid (tannin) which on hydrolysis yields Gallic acid The Gallic acid present in Amla fruit has antioxidant property. The retention of Vitamin-C in Amla products due to this polyphenol is a matter of great concern for processors.
Amla is a perishable commodity, needs its quick disposal. Though Amla fruit is being tried and processed in the preparation of different products like pulp, preserve, candy, jam, syrup, shreds, pickles, squash and sauce, due to its attractive flavour, acidity and astringency attributes it can also be used in the preparation of alcoholic beverage (wine). In dearth of information for finding out a suitable methodology. an experiment was carried out at State Institute of Food Processing Technology, Lucknow (U.P.) to study the enological qualities of Amla fruit.

MATERIALS AND METHODS
Amla fruits of var. Banarasi were procured from the Horticultural Gardens of District Pratapgarh (U.P.). Harvesting of fruit was done in the I” week of January. Fruits of identical size and age were randomly harvested from four different branches of ten different trees and mixed uniformly for experimental use.
The trial was carried out at Microbiology Laboratory, State Institute of Food Processing Technology. Lucknow (U.P.).
Physical observations were recorded with twenty fruits taken from the above randomised lot For chemical estimation composite samples of ten fruits were prepared in four replications. The data recorded are reported as mean of four replications.
PREPARATION OF STARTER
Starter was prepared by making a malt solution by adding 20% sugar, 0.03% tartaric acid, 2% malt extract, 2% yeast extract, 0.2% citric acid and 0.2% Ammonium phosphate in distilled water. It was just boiled and inoculated with 7 days old culture of Saccharomyces cerevisiae var. ellipsoideus burgundy on M.G.Y.P. medium. A vigorous fermentation was observed after 18 hours in this solution and it was used as starter
PREPARATION OF MUST
The Amla fruits of Banarasi cultivar were washed in running water thoroughly and grated with hand grater. Pulp was divided in five lots. In a preliminary experiment fermentation could not be run successfully with the pure pulp, with pure juice, juice with increased pH by adding sodium bi carbonate and with diluted pulp by adding 10% boiling water. So 20%, 30%, 40%, 50% and 60% boiling water was poured over on pulp of 14, 2nd, 3rd, 4th and 5th lots respectively to kill naturally occurring strain of yeasts and bacteria on fruits and also to reduce the high acid and tannin contents of the fruits. (By dilution effect) in order to produce a palatable wine. T.S.S. of the each lot was measured and cane sugar was ameliorated to bring the T.S.S. at 23°Brix level as given in the following table:-
T1- Amla Pulp 80% + Boiling water 20%
T2:- Amla Pulp 70% + Boiling water 30%
T3 – Amla Pulp 60% + Boiling water 40%
T4-Amla Pulp 50% + Boiling water 50%
T5-Amla Pulp 40% + Boiling water 60%
SO₂ was added at the rate of 100 ppm in the form of potassium meta bisulphite. After half an hour must was inoculated with starter at the rate of 4 percent and fermentation was set at room temperature (25- 27°C).
After 5 days of vigorous fermentation all the lots were pressed through muslin cloth and the pomace was discarded.
All lots were subjected to fermentation till almost all the sugar was consumed (to dryness). After settling the wines were racked into clean sterilized glass bottles. After racking bottles were sealed and stored at room temperature (25-35°C) for maturation. The wines were racked at intervals of two months for a period of six months.
FLOW SHEET FOR PREPARATION OF AMLA WINE
Ripe Amla Fruit
Washing
Grating (Pulping)
Dilution by adding boiling water
Addition of sugar
Pasteurization at 82°C for 2 minutes
Cooling
Addition of SO₂ (100 ppm)
Addition of Starter (4%)
Fermentation
Siphoning
Aging (Maturation)
Racking (at intervals of two months)
Bottling
Pasteurization
Cooling
Storage
DETAIL METHODS OF ANALYSIS OF FRUIT, MUST AND WINE ARE AS FOLLOWS:-
PHYSICAL PARAMETERS OF AMLA FRUIT
LENGTH
The length of twenty Amla fruits were measured separately by Vernier Callipers. Two values were taken consequently and finally
average value was obtained.
DIAMETER
Diameter of twenty Amla fruits were measured separately by Vernier Callipers. Taking two observations the average values were obtained
CIRCUMFERENCE
The circumference of fruit of each lot was measured with the help of tension free cotton thread.
WEIGHT
Weight of twenty Amla fruits were recorded separately in each replication by laboratory physical balance.
FIRMNESS & SOFTNESS
Firmness & softness of the fruit was measured by Megnus Taylor Pressure tester fitted with plunger of 5/16″.
SPECIFIC GRAVITY
Volume of the twenty Amla fruits were determined by water displacement method. A measuring cylinder of one litre capacity was taken and filled with water. Volume of fruit was measured by rise in water level. Specific gravity of Amla fruit was measured by dividing the weight of Amla in the air by volume of fruit.
CHEMICAL PARAMETERS OF AMLA FRUIT AND MUST
- pH
pH of the sample was determined by glass electrode pH meter (A.O.A.C., 1970).
- TOTAL SOLUBLE SOLIDS
Total soluble solids in the juice of the Amla fruit was determined by hand refractometer (Ranganna 1986). Firstly the Amla was washed, cleaned and thereafter crushed to extract the juice. For determining the total soluble solids, the blended sample was placed between the prism of refractometer and reading was noted after zero correction.
- ACIDITY
Acidity of Amla fruit was determined by simple acid-base titration method using phenolphthalein as an indicator (A.O.A.C.. 1970).
For calculating the acidity, 10 gms of Amla fruit was taken, crushed in a pastel-motel and the volume finally was made upto 250 ml and filtered by using a piece of cotton. 20 ml of the filtered sample solution was titrated against N/10 NaOH.
Using phenolphthalein as an indicator till the appearance of light pink colour which persists for 30 seconds. The value of NaOH used was noted and the acidity was calculated in terms of citric acid.
- REDUCING, NON REDUCING AND TOTAL SUGAR
(a) ESTIMATION OF REDUCING SUGAR
Sugar in the sample was estimated By Lane and Eynon method (1923). After washing the fruit 10 gm of it was pulped by a homogeniser in a blender using distilled water and finally its volume was made upto 250ml mark. 5ml of each Fehling’s solution (Fehling A and Fehling B) were taken in a conical flask 20ml of water was added to its contents, were then heated on gas burner having a wire gauge. It was heated up to boiling To the boiling Fehling’s solution sample solution, was added drop wise with the help of burette till appearance of pale red colour, 2-3 drops of methylene blue indicator was added drop wise End point was indicated by the decolourisation of indicator and appearance of brick red colour due to precipitation of cuprous oxide. The volume of solution required was noted for the evaluation of reducing sugars.
(b) ESTIMATION OF TOTAL SUGARS (AS INVERT SUGAR)
For estimation of total reducing sugars, 50 ml of clarified solution prepared for the estimation of reducing sugar 10 ml of hydrochloric acid was added in it and allowed to stand for 24 hours. This was neutralized with sodium bicarbonate and made upto 100 ml mark. The sample is taken to determine the total reducing sugar as invert sugar.
The reducing sugar, non reducing sugar and total sugar was calculated following factors:-
a. % Reducing Sugars
Mg. Of Invert sugar x Dilution x 100
Titre x Wt or volume of the sample x 100
b. % Total Sugars as invert sugar Calculate as in (a)
Making use of the titre value obtained in determination of the total sugars after inversion.
c. % Non Reducing sugar (Sucrose) = (% Total Invert Sugars – % Reducing Sugars originally present) x0.95
d. % Total Sugars = (% Non Reducing Sugar (Sucrose) + % Reducing sugars)
- PECTIN (AS CALCIUM PECTATE)
For the estimation of pectin as calcium pectate the pectin extracted from the pulp was saponified with alkali and was precipitated as calcium pectate from an acid solution by CaCl₂ The calcium precipitate is washed until free from chloride Dried and weighed, (Ranganna, 1986)
50gms of blended pulp of Amla was taken into 1000 ml beaker and pectin was extracted with 400 ml of 0.05N Hydrochloric acid keeping it for two hours at 80°C-90°C water loss by evaporation was fulfilled. After cooling transferred the content to a 500ml volumetric flask and make with water. Shaken and filtered through No 4 Whatman filter paper into 500 ml conical flask, pipetted 100ml aliquot each into 1000ml beaker. Added to it 250ml of water and neutralized the acid with IN sodium hydroxide using phenolphthalein as indicator. 10ml of IN sodium hydroxide was added in excess with continuous stirring and allowed the suspension to stand for over night.
Added 50ml of IN acetic acid to it and after 5 minutes added 25ml of IN calcium Chloride solution with stirring. After allowing it to stand for one hour Boiled for 1 or 2 minutes. Filtered through previously prepared filter paper. The filter paper was prepared by drying in oven at 102°C for two hours, cooled in dessicator was washed the precipitate with boiling water until it was free from chloride ion. Chloride ion was tested using silver nitrate as an indicator.
Transferred the filter paper containing the calcium pectate to the original weighing dish, dried overnight at 100°C cooled in the dessicator and weighed to a constant value. The pectin content was calculated as calcium pectate.
Wt of Calcium Pectate x 500 x 100 ml of filtrate x wt. of sample %Pectin (as Calcium Pectate) =
- ASCORBIC ACID
Ascorbic acid was estimated by reduce a standard dye solution as determined by titration, (Ranganna, 1986).
For dye solution 50mg of 2,6-dichlorophenol indophenol was dissolved in 150ml of hot distilled water containing 42mg sodium carbonate and then dilute with distilled water upto 200 ml 3% metaphosphoric acid (HPO₁) solution was prepared. Standard ascorbic acid solution was prepared by dissolving 100mg of L ascorbic acid in 3% metaphosphoric acid solution upto 100 ml titrates the dye solution with standard ascorbic acid to standardize the dye solution, till pink colour persists for 15 seconds
Dye factor was calculated as
Dye factor (D.F.) =
Titre
10ml of sample was taken and made upto 100ml with 3% HPO). Then sample was titrated with the standard dye to pink end point. Ascorbic acid present in sample was calculated by the following formula-
Ascorbic acid = (mg/100gm) Titre x Dye factor x volume made up x 100 Vol. Of Filtrate taken wt. or Vol. of sample taken
- TANNINS (AS TANNIC ACID)
Prepared Folin-Denis reagent by adding 100gm of sodium tungstate [Na₂WO,2H₂O], 20gms of phosphomolybdic acid and 50 ml of phosphoric acid to 750 ml of water, reflux two hour and dilute to one litre. Also prepared a saturated solution of sodium carbonate by adding 35 gms. of anhydrous sodium carbonate in 100 ml of water at about 176°F (80°C) also prepared standard tannic acid solution by dissolving 100 mg of tannic acid in a litre of water
Pipetted one ml of juice into a nesslar tube containing 80 ml of water. Added 5 ml of the Folin-Denis reagent and 10 ml of the sodium carbonate solution and shaken well and made upto the mark After 30 minutes compared the colour to that developed with standard tannic acid solutions prepared in the same way. Standards containing 0.0, 0.2, 0.4, 0.6, 0.8, 1.0, 12, 1.4, 1.6, 1.8, 2.0, 2.2, 2.4, 2.6, 2.8, 3.0 ml of the standard tannic acid solution are useful
(Amerine and Cruess) 1960
ANALYSIS OF AMLA WINE
- ALCOHOL
Alcohol was determined by specific gravity of distillate from measured volume of wine. This method was based on regular variation of specific gravity in mixture of water and alcohol (Α.Ο.Α.C., 1970).
A small distilling apparatus of a 500 ml round bottom flask and connected with a condenser both attached to the stand An electric hot plate was used for the heating of the flask. Pipetted 100 ml of wine into the distilling flask and added 50 ml of distilled water. Start the cold water flow through condenser Heated the sample to boiling and boiled until 100 ml. of distillate is collected in the volumetric flask.
Weighed the pyenometer when dry and empty Filled the pycnometer with distilled water at the standard temperature (60°F) 15.5°C. Inserted the thermometer and brought the contents at standard temperature. Weighed again accurately. Now emptied and dried the pycnometer, rinsed the pycnometer with a little distillate and then filled it with distillate. Cooled the distillate and adjusted the temperature of the distillate to that of distilled water. Wiped the pycnometer, dried with a clean soft cloth. Put the overflow tube’s cup in place and weighed accurately. Specific gravity of distillate was calculated by the following formula.
Specific gravity of distillate = Weight of distillate Weight of same volume of distilled water.
The alcohol percentage by volume was found out with the help of standard specific gravity alcohol percentage table as given in A.O.A.C. (1970).
- ESTER
Ester in the wine was determined as ethyl acetate by the method of Joslyn and Amerine (19416). Pipetted 100 ml of wine and about 35 ml of water in to 500 ml distillation flask. At last 50 ml was distillated at a maximum heat to assure distillation of all the furfural. The receiving tube from the condenser should reached well into the receiving flask. Electric heater was used to avoid local over heating and to avoid furfural formation. 100 ml of distillate was taken in 500 ml. round bottom flask and neutralized the free acid present with 0.IN NaOH, using phenolphthalein as an indicator. Then added a measured excess amount of 0. IN NaOH (25 ml) and connect the flask to a reflux condenser and boiled for one hour. After cooling the flask 25 ml of 0. IN HCI was added and then titrated to a pink end point with 0.1 N NaOH. The amount of 0.1N alkali added minus the amount of 0. IN HCI used for the final titration gives the amount of 0.IN alkali required to saponify the ester present. The ester as ethyl acetate was calculated by following factor:-
The amount of 0.IN NaOH used x 8.8 = mg. of Ester as ethyl acetate in 100 ml of wine
- VOLATILE ACIDITY
Took 100 ml of wine into a round bottom distillation flask refluxed it for about 15 minutes so that all the carbon dioxide present in wine was removed. Then cooled the flask, poured 50 ml water in that flask and heated the flask for distillation. Collected exactly 100 ml distillate in to a flask. 3-4 drops of phenolphthalein was added to it and titrated to a definite pink colour with the N/10 NaOH. Calculated the volatile acidity, as I ml N/10 NaOH is equal to 0.0060 g of acetic acid. (Amerine & Cruess, 1960)
- TOTAL ACIDITY
Pipetted 5 ml of wine into 250 ml Erlenmeyer flask and added 75 ml of boiling water and 5 drops of phenolphthalein. Slowly added 0.1N in a sample solution by burette until the colour changes to pink, the amount of alkali utilized was noted The total acidity as citric acid per 100 ml was calculated as follows; (Amerine and Cruess; 1960)
1 ml of N/10 = 0.0064 gms of citric acid
- UNFERMENTED SUGAR
Unfermented sugar in wine was estimated by the method as described by (Amerine and Cruess; 1960)
- pH
The pH of Amla Wine was determined by glass electrode pH meter. (A.O.A.C.; 1970).
- TOTAL SOLUBLE SOLIDS
The total soluble solids are present in wine was determined by hand refractometer. (Ranganna; 1986).
- TANNINS
The tannins present in wine was estimated as tannic acid by the method as described by Amerine and Cruess (1960) estimated as earlier.
- RIPPER METHOD OF TOTAL SULPHUR DIOXIDE
This is a volumetric method based on direct titration of Sulphur dioxide in the wine after hydrolysis of the combined Sulphur dioxide by strong alkali.
Checked the normality of the 0.02N lodine solution by pipetting 25ml into 50 ml of the distilled water in a 300c.c. Erlenmeyer flask. Add 10ml of the dilute sulphuric acid and a few drops of starch solution. From a burette added exactly O. IN thiosulphate until one drop finally destroys the blue colour. The normality of the Iodine solution is then obtained by the following calculation:-
(ml. Sodium Thiosulphate x 0.10)/25 = Normality of lodine.
Now pipetted out 20 ml of the wine into a 250 ml Erlenmeyer flask. Added 25ml of 10% NaOH, mixed properly, and allowed to stand exactly 15 minutes. Then added 10 ml of dilute sulphuric acid solution and 5 ml of starch solution as an indicator. Titrated immediately to a permanent blue colour with the 0.02N Iodine.
The calculation was based on the fact that :-
Iml of exactly 0. IN iodine used x normality of iodine =p.p.m. of sulphur dioxide
- PECTIN
Pectin to be estimated was saponified with alkali and precipitated as calcium pectate from an acid solution by the addition of calcium chloride. The method was same as described earlier. (Ranganna, 1960)
- ORGANOLEPTIC EVALUATION
A panel of 5 judges carried out organoleptic evaluation of wine prepared by different treatments. The evaluation was made on the score card as suggested by Amerine and Cruess (1960)
CHEMICALS USED IN ANALYSIS
For analysis work only distilled water was used, which was prepared by double glass distillation process. The chemicals which were prepared by double glass distillation process. The chemicals which are used during the experiment were analytical reagents of BDH and Glaxo product.

RESULTS & DISCUSSION
Physical characters and chemical composition of fresh fruit of Amla are given in Table-2 and Table-3 respectively
TABLE-2
PHYSICAL PARAMETERS OF RIPE AMLA FRUIT
CHARACTERS VALUES
Colour : Yellowish green
Length (Cm.) : 3.01
Diameter (Cm.) : 3.43
Average Circumference (Cm.) : 13.68
Average Weight (gm.) : 37.782
Firmness and Softness(lb/sq. inch) : 18.20
Specific Gravity : 1.0331
TABLE-3
CHEMICAL COMPOSITION OF FRESH AMLA FRUIT
PARTICULARS : VALUES
T.S.S (Brix) : 14
pH : 1.82
Acidity (As % Citric Acid) : 1.76
Reducing Sugars (%) : 5.56
Non Reducing Sugars (%) : 1.92
Total Sugars (%) 7.48
Ascorbic Acid (mg/100gm) : 650
Pectin (As % Calcium Pectate) : 0.54
Tannin (as Tannic Acid in g/100gm): 2.80
Total Soluble Solids (T.S.S.) was found at 14 °Brix, pH at 1.82 level, titrable acidity as citric acid 1.76 percent, ascorbic acid 650 mg/100 gm, reducing sugars 5.56%, non reducing sugars 1.92%, total sugars 7.48%, pectin as calcium pectate 0.54% and tannins as tannic acid was found 2.80 gm/100gm.
As the acid and tannin contents in Amla fruits were high, boiling water was added in 80:20, 70:30, 60:40, 50:50 and 40:60 ratio into the fruit pulp to get good fermentation and a palatable wine. The diluted musts were ameliorated with cane sugar to bring the Brix at 23° level before setting for fermentation in order to get the desired level of alcohol. Schanderl and Koch, also recommended the amelioration of berries and plum with water and cane sugar to produce a palatable wine. Dilution and addition of cane sugar resulted in decrease of all the constituents in the musts to an appreciable level (Table-4), except the pH which increased in range 1.88 to 2.23. The acidity decreased in range 1.400 to 0.698 percent, Ascorbic acid decreased 520 to 270 mg/100gm, Pectin decreased 0.432 to 0.216 percent and tannin decreased 2.24 to 1.12 g/100gm in diluted lots (T1, T2, T3, T4 and T5).
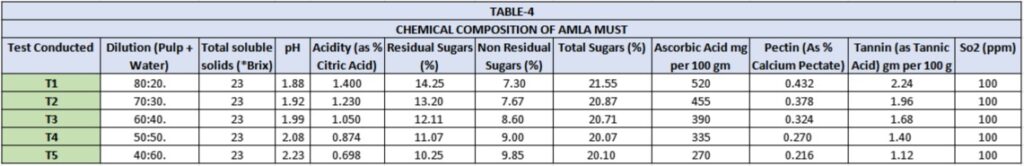
The yield and composition of aged wine are presented in Table-5 The alcohol content in wine lots T1, T2 and T1, was found low (5.62, 7.24 and 8.23 percent) while residual sugar was high (6.88, 3.98 and 2.81 gm/100ml respectively), in contrast to lots T4 and T5 where alcohol contents were found high (9.22 and 10.54 percent respectively) and residual sugar 2.12 and 1.08gm/100ml respectively. This clearly indicated that fermentation went on for some time even during maturation.
The wine lots T4 and T5 could be classified as dry wines.
Dilution of pulp of different lots decreased the acid and tannın contents and it helped the carrying of fermentation.
Total acidity in matured wine ranged from 0.56 to 1.12 gm/100ml which is slightly less than the acidity of the must. Volatile acidity (as acetic acid) ranged from 0.028 to 0.035 gm/100ml. Ascorbic acid ranged from 108 to 208 mg/100ml. Ester (as ethyl acetate) ranged from 14.24 to 20.48 mg/100ml. An appreciable decrease was found in tannin contents which now ranged from 160 to 400 mg/100ml respectively. The possible reason of decrease of these polyphenolic compounds may be due to their combination with aldhydes, to precipitate with added or natural proteins and to other reactions (Amerine and Cruess, 1960). The pectin contents were found very much decreased and found nil in all lots. This may be due to the precipitation of pectin with alcohol produced.
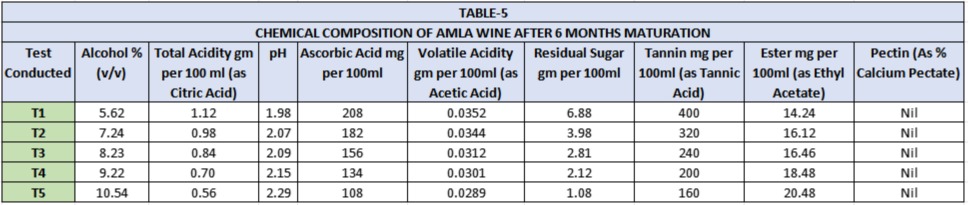
The organoleptic evaluation was done by a panel of 5 judges (Table-6). Clarity, colour, bouquet and taste are the characters responsible for acceptance or rejection of any wine. In sensory evaluation the wines secured 48 to 76 percent marks Among T1, T2, T3, T4 and T5 lots. Wine of T5 (40:60 dilution) was adjudged best as it secured maximum (76) marks. It gave wine of more balanced acid, tannin and colour contents and also produced higher alcohol yield. It was closely followed by lot T4 (50:50 dilution). Thus dilution of pulp was found necessary for making Amla wine.
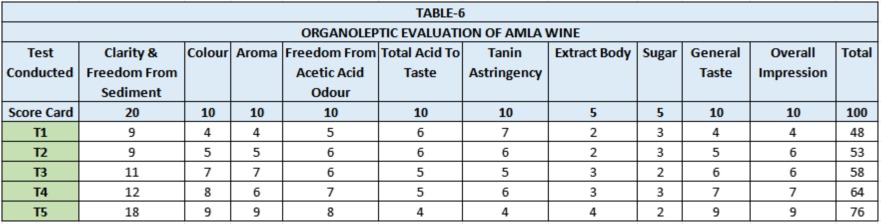
(Average Score of five judges)

SUMMARY
In an experiment to find out a methodology for wine making from Amla fruits, wine was prepared by adding boiling water in 20, 30, 40, 50 and 60 percent ratio into the fruit pulp in T1, T2, T3, T4, and T5 lots respectively.
These lots were ameliorated with cane sugar for maintaining a T.S.S. level at 23°Brix. These were fermented by the addition of 4% starter prepared from Sacchromyces cerevisiae. After fermentation, wine lots were put for maturation for a period of six months. Chemical analysis and organoleptic evaluation of the matured wine showed that dilution of fruit pulp (40:60) gave a wine of more balanced acid and tannin contents and a higher alcohol yield. It was closely followed by 50 50 dilution lot.
PERFORMANCE
It clearly indicates that dilution of Amla pulp is necessary for making a quality wine.





Great post however , I was wanting to know if you could write a litte more on this topic? I’d be very grateful if you could elaborate a little bit further. Bless you!
As I website owner I believe the content material here is very good, regards for your efforts.
Hi there very cool blog!! Guy .. Beautiful .. Wonderful .. I’ll bookmark your web site and take the feeds also…I’m happy to search out a lot of helpful info here within the publish, we need develop more techniques in this regard, thanks for sharing. . . . . .
hi!,I really like your writing so a lot! proportion we keep up a correspondence extra about your article on AOL? I need a specialist in this space to solve my problem. May be that is you! Looking ahead to see you.
Thank you for sharing excellent informations. Your site is so cool. I am impressed by the details that you have on this blog. It reveals how nicely you understand this subject. Bookmarked this website page, will come back for extra articles. You, my pal, ROCK! I found just the info I already searched everywhere and simply could not come across. What a perfect website.
Aw, this was a very nice post. In concept I wish to put in writing like this moreover – taking time and precise effort to make a very good article… however what can I say… I procrastinate alot and certainly not appear to get something done.
I am always browsing online for ideas that can help me. Thank you!
Good write-up, I’m regular visitor of one’s blog, maintain up the nice operate, and It is going to be a regular visitor for a lengthy time.
Thanks for another wonderful article. Where else could anyone get that kind of info in such an ideal way of writing? I’ve a presentation next week, and I’m on the look for such information.
You actually make it appear really easy along with your presentation but I find this topic to be actually one thing that I believe I’d by no means understand. It sort of feels too complex and extremely huge for me. I’m looking ahead for your next post, I?¦ll try to get the hold of it!
great points altogether, you just received a emblem new reader. What would you suggest about your put up that you simply made a few days in the past? Any sure?
I was suggested this blog by my cousin. I am not sure whether this post is written by him as no one else know such detailed about my difficulty. You’re incredible! Thanks!
I see something really special in this site.
I’ve been browsing on-line greater than 3 hours these days, yet I never discovered any attention-grabbing article like yours. It is pretty price sufficient for me. In my view, if all site owners and bloggers made excellent content as you did, the internet will probably be much more useful than ever before.
Hi there! I just wanted to ask if you ever have any trouble with hackers? My last blog (wordpress) was hacked and I ended up losing several weeks of hard work due to no backup. Do you have any solutions to prevent hackers?
I have been surfing online more than three hours nowadays, but I never discovered any attention-grabbing article like yours. It’s lovely value sufficient for me. In my opinion, if all webmasters and bloggers made excellent content as you probably did, the net will likely be much more helpful than ever before.
Its like you read my mind! You seem to know a lot about this, like you wrote the book in it or something. I think that you could do with some pics to drive the message home a little bit, but instead of that, this is wonderful blog. An excellent read. I’ll definitely be back.
fantastic post, very informative. I wonder why the other experts of this sector don’t notice this. You must continue your writing. I am sure, you’ve a great readers’ base already!
Enjoyed studying this, very good stuff, thanks. “We swallow greedily any lie that flatters us, but we sip little by little at a truth we find bitter.” by Denis Diderot.
I have read a few good stuff here. Definitely worth bookmarking for revisiting. I surprise how much effort you put to make such a magnificent informative website.
I don’t commonly comment but I gotta tell appreciate it for the post on this perfect one : D.
I have read some good stuff here. Definitely worth bookmarking for revisiting. I surprise how a lot attempt you set to make such a great informative site.
Oh my goodness! an incredible article dude. Thank you Nonetheless I’m experiencing problem with ur rss . Don’t know why Unable to subscribe to it. Is there anyone getting identical rss downside? Anybody who is aware of kindly respond. Thnkx
I have been absent for some time, but now I remember why I used to love this blog. Thank you, I’ll try and check back more frequently. How frequently you update your web site?
Great web site. A lot of useful information here. I am sending it to some buddies ans also sharing in delicious. And obviously, thank you to your sweat!
I discovered your blog site on google and check a few of your early posts. Continue to keep up the very good operate. I just additional up your RSS feed to my MSN News Reader. Seeking forward to reading more from you later on!…
hi!,I really like your writing so so much! percentage we communicate more about your post on AOL? I need a specialist in this space to unravel my problem. Maybe that is you! Having a look forward to peer you.
This web site is my inhalation, very excellent design and style and perfect articles.
I am continuously browsing online for posts that can help me. Thank you!
I like the helpful info you provide in your articles. I’ll bookmark your blog and check once more here regularly. I’m moderately certain I’ll learn many new stuff proper here! Good luck for the next!
Hello. impressive job. I did not anticipate this. This is a great story. Thanks!
You made several good points there. I did a search on the subject and found most folks will consent with your blog.